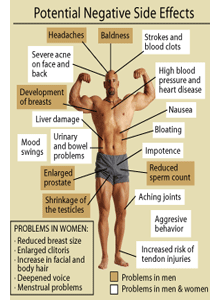
While anabolic/androgenic steroids (AAS) are generally regarded as therapeutic drugs with high safety, their use can also be associated with a number of adverse cosmetic, physical, and psychological effects. Many of these side effects are often apparent during therapeutic-use conditions, although their incidence tends to increase profoundly as the dosages reach supratherapeutic ranges. Virtually everyone that abuses anabolic/androgenic steroids for physique-or performance-enhancing purposes notices some form of adverse effects from their use. According to one study, the exact frequency of tangible side effects in a group of steroid abusers was 96.4%. This shows very clearly that it is far more rare to abuse these drugs and not notice side effects than it is to endure them.88 In addition to the side effects that anabolic/androgenic steroids can have on various internal systems, there are others which may not be immediately apparent to the user. The following is a summary of the biological systems and reactions effected by AAS use.
Cardiovascular System
The use of anabolic/androgenic steroids in supratherapeutic (and often therapeutic) doses can have a number of adverse effects on the cardiovascular system.
\ This may be noticed in several areas including unfavorable alterations in serum cholesterol, a thickening of ventricular walls, increased blood pressure, and changes in vascular reactivity. In an acute sense these drugs are admittedly very safe. The risk of an otherwise healthy person suffering a heart attack from an isolated steroid cycle is extremely remote. The risk of stroke is also extremely low. When these drugs are abused for long periods, however, their adverse effects on the cardiovascular system are given time to accumulate. An increased chance of early death due to heart attack or stroke is, likewise, a valid risk with long-term steroid abuse. In order to better understand this risk, we must look specifically at how anabolic/androgenic steroids affect the cardiovascular system in several key ways.
Cholesterol/Lipids
Anabolic/androgenic steroids use can adversely affect both HDL (good) and LDL (bad) cholesterol values. The ratio of HDL to LDL cholesterol fractions provides a rough snapshot of the ongoing disposition of plaque in the arteries, either favoring atherogenic or anti-atherogenic actions. The general pattern seen during steroid use is a lowering of HDL concentrations, which is often combined with stable or increased LDL levels.Triglyceride levels may also increase.The shift can be unfavorable in all directions. Note that in some cases, the total cholesterol count will not change significantly. The total cholesterol level can, therefore, give a false representation of uncompromised lipid health. Almost invariably the underlying HDL/LDL ratio will decrease.While this ratio should return to normal following the cessation of steroid intake, plaque deposits in the arteries are more permanent. If unfavorable shifts in lipids are exacerbated by the long-term use of steroidal compounds, significant damage to the cardiovascular system can result.
Anabolic/androgenic steroids are most consistent in their lowering of HDL levels. This adverse effect is mediated through the androgenic stimulation of hepatic lipase, a liver enzyme responsible for the breakdown of HDL (good) cholesterol.89 With more hepatic lipase activity in the body, the favorable (anti-atherogenic) HDL cholesterol particles are cleared from circulation more quickly, and their levels drop. This is an effect that seems to be very pronounced at even modest supratherapeutic dosage levels. For example, studies with testosterone cypionate noted a 21 % drop in HDL cholesterol with a dosage of 300 mg per week.90 Increasing this dosage to 600 mg did not have any significant additional effect, suggesting that the dosage threshold for strong HDL suppression is fairly low.
Oral steroids, especially c-17 alpha alkylated compounds, are particularly potent at stimulating hepatic lipase and suppressing HDL levels. This is due to first pass concentration and metabolism in the liver. A drug like stanozolol may, therefore, be milder than testosterone with regard to androgenic side effects, but not when it comes to cardiovascular strain. A study comparing the effects of a weekly injection of 200 mg testosterone enanthate to only a 6mg daily oral dose of stanozolol demonstrates the strong difference between these two types of drugs very wel1.91 After only six weeks, 6mg of stanozolol was shown to reduce HDL and HDL-2 cholesterol levels by an average of 33 and 71 % respectively. HDL levels (mainly the HDL-3 subfraction) were reduced by only 9% in the testosterone group. LDL cholesterol levels also rose 29% with stanozolol, while they dropped 16% with testosterone. Esterified injectable steroids are generally less stressful to the cardiovascular system than oral agents.
It
is also important to note that estrogens can have a favorable impact on cholesterol profiles. The aromatization of testosterone to estradiol may, therefore, prevent a more dramatic change in serum cholesterol. A study examined this effect by comparing the lipid changes caused by 280 mg of testosterone enanthate per week, with and without the aromatase inhibitor testolactone.92 Methyltestosterone was also tested in a third group, at a dose of 20 mg daily, to judge the comparative effect of an oral alkylated steroid. The group using only testosterone enanthate in this study showed a small but not significant decrease in HDL cholesterol values over the course of the 12-week investigation. After only four weeks, however, the group using testosterone plus the aromatase inhibitor displayed an HDL reduction of an average of 25%. The group taking methyltestosterone experienced the strongest HDL reduction in the study, which dropped 35% after four weeks. This group also noticed an unfavorable rise in LDL cholesterol levels.
The potential positive effect of estrogen on cholesterol values also makes the issue of estrogen maintenance something to consider when it comes to health risks. To begin with, one may want to consider whether or not estrogen maintenance drugs are actually necessary in any given circumstance. Are side effects apparent, or is their use a preventative step and perhaps unnecessary? The maintenance drug of choice can also have a measurable impact on cholesterol outcomes. For example, the estrogen receptor antagonist tamoxifen citrate does not seem to exhibit anti-estrogenic effects on cholesterol values, and in fact tends to increase HDL levels in some patients. Many individuals decide to use tamoxifen to combat estrogenic side effects instead of an aromatase inhibitor for this reason, particularly when they are using steroids for longer periods of time, and are concerned about their cumulative cardiovascular side effects.
Enlarged Heart
The human heart is a muscle. It possesses functional androgen receptors, and is growth-responsive to male steroid hormones. This fact partly accounts for men having a larger heart mass on average than women.93 Physical activity can also have a strong effect on the growth of the heart. Resistance exercise (anaerobic) tends to increase heart size by a thickening of the ventricular wall, usually without an equal expansion of the internal cavity. This is known as concentric remodeling. Endurance' (aerobic) athletes, on the other hand, tend to increase heart size via expansion of the internal cavity, without significant thickening of the ventricles (eccentric remodeling). Even with concentric or eccentric remodeling, diastolic function usually remains normal in the athletic heart.The heart muscle is also dynamic.When regular training is removed from a conditioned athlete, the wall thickening and cavity expansion tend to reduce.
Anabolic steroid abusers are at risk for thickening of the left and right ventricular walls,94 known as ventricular hypertrophy. Hypertrophy of the left ventricle (the main pumping chamber) in particular is extensively documented in anabolic/androgenic steroid abusers.951 While left ventricular hypertrophy is, again, also found in natural power athletes, substance-abusing athletes tend to have a much more profound wall thickening. They also tend to develop pathological issues related to this thickening, including impaired diastolic function, and ultimately reduced heart efficiency.96 The level of impairment is closely associated with the dose and duration of steroid abuse. A left ventricle wall exceedinc 13mm in thickness is rare naturally, and may be indicativ~ of steroid-abuse or other causes.97 It may further sugges1 that pathological left ventricular hypertrophy ha~ developed. Additional testing of such patients d recommended.
Left ventricular hypertrophy (LVH) is an independenl predictor of mortality in overweight individuals with hig( blood pressure.98 It has also been linked to atri~ fibrillation, ventricular arrhythmia, and sudden collaps! and death.99 While LVH in non-steroid-using athlete' tends to be without clinical significance, pathologicc increases in QT dispersion are noticed in steroid abuse~i ~ith LVH ..100 The.se ch~nges ten.d to be si~ilar to th~ Increases In QT dispersion noted In hypertensive patientl with LVH.l0l Among other things, this could leave a steroi~ abusing individual more susceptible to a serious advers~ event, including arrhythmia or heart attack. Isolate~ medical case studies of longtime steroid abusers suppOr] an association between LVH and related pathologieJ including ventricular tachycardia (arrhythmia originatind in the left ventricle), left ventricular hypokinesil,(weakened contraction of the left ventricle), and decreased ejection fraction (reduced pumping volume and efficiency)
Heart mass can increase or decrease in relation to the current state of anabolic/steroid use, the average dosage, and duration of intake. Likewise, the heart usually begins to reduce in size once anabolic/androgenic steroids are no longer being used. This effect is similar to the way the heart will reduce in size once an athlete no longer follows a rigorous training schedule.103 Even with this effect, however, some changes in heart muscle size and function caused by the drugs may persist. Studies examining the effects of steroid use and withdrawal on left ventricular hypertrophy noted that athletic subjects who abstained from steroid abuse for at least several years still had a slightly greater degree of concentric left ventricular hypertrophy compared to non-steroid-using athletic controls.104 The disposition of pathological left ventricular hypertrophy following long-term steroid abuse and then abstinence remains the subject of investigation and debate.
Heart Muscle Damage
Anabolic/androgenic steroid abuse is suspected of producing direct damage to the heart muscle in some cases. Studies exposing heart cell cultures to AAS have reported reduced contractile activity, increased cell fragility, and reduced cellular (mitochondrial) activity, providing some support for a possible direct toxic effect to the heart muscle.10s 106 Furthermore, a number of case reports have found such pathologies as myocardial fibrosis (scar tissue buildup in the heart), myocardial inflammation (inflammation of heart tissue), cardiac steatosis (accumulation of triglycerides inside heart cells), and myocardial necrosis (death of heart tissue) in longterm steroid abusers.10? 108 109 110 A direct link between drug abuse and cardiac pathologies is assumed in these cases, but cannot be proven given the slow nature in which these cardiac pathologies develop, and the influence many other factors (such as diet, exercise, lifestyle, and genetics) can have on them. Individuals remain cautioned about the possibility of cardiac muscle damage with long-term steroid abuse.
taking steroids.112 Hypertension, or consistently high blood pressure at or above 140/90 for either systolic or diastolic measures, has been reported in steroid users/113 although in most cases the elevations are more modest. Increased blood pressure may be caused by a number of factors, including increased water retention, increased vascular stiffness, and increased hematocrit. Aromatizing or highly estrogenic steroids tend to cause the greatest influences over blood pressure, although elevations cannot be excluded with non-estrogenic anabolic/androgenic steroids. Blood pressure tends to normalize once anabolic/androgenic steroids have been discontinued.
Hematological (Blood Clotting)
Anabolic/androgenic steroids can cause a number of changes in the hematological system that affect blood clotting. This effect can be very variable, however. The therapeutic use of these drugs is known to increase plasmin, antithrombin 111/ and protein S levels, stimulate fibrinolysis (clot breakdown), and suppress clotting factors II, V, VII, and ,X.114 115 These changes all work to reduce clotting ability. Prescribing guidelines for anabolic/androgenic steroids warn of potential increases in prothrombin time, a measure of how long it takes for a blood clot to form.116 If prothrombin time increases too greatly, healing may be impaired. The effects of anabolic/androgenic steroids on prothrombin time are generally of no clinical significance to healthy individuals using these drugs in therapeutic dosages. Patients taking anticoagulants (blood thinners), however, could be adversely affected by their use.
Conversely, anabolic/androgenic steroid abuse has been linked to increases in blood clotting ability. These drugs can elevate levels of thrombin 11 ? and C-reactive protein,118 as well as thromboxane A2 receptor density,119 which can support platelet aggregation and the formation of blood clots. Studies of steroid users have demonstrated statistically significant increases in platelet aggregation values in some subjects.120 There are also a growing number of case reports where (sometimes fatal) blood clots, embolisms, and stokes have occurred in steroid abusers. Although it has been difficult to conclusively link these events directly to steroid abuse, the adverse effects of anabolic steroids on components of the blood coagulation system are well understood. These serious adverse effects are now regarded as recognized risks of steroid abuse among many that study these drugs. In therapeutic levels, the anti-thrombic effects of anabolic/androgenic steroids seem to dominate physiology, and decreases in blood clotting ability may be noted. At a certain supratherapeutic dosage point, however, the pro-thrombic changes appear to overtake the anti-thrombic changes, and physiology begins to favor fast and abnormally thick clot formation (hypercoagulability). The exact dosage threshold or conditions required to increase blood clotting has not been determined, and some studies with steroid users taking supraphysiological doses fail to demonstrate increased coagulability.126 Individuals remain warned of the potential increases in thrombic risk with anabolic/androgenic steroid abuse. Blood clotting tendency should return to the pretreated state after the discontinuance of anabolic/androgenic steroids.
Hematological {Polycythenlial
Anabolic/androgenic steroids stimulate erythropoiesis (red blood cell production). One potential adverse effect of this is polycythemia, or the overproduction of red blood cells. Polycythemia can be reflected in the hematocrit level, or the percentage of blood volume that is made up of red cells. As the hematocrit rises, so too does the viscosity of the blood. If the blood becomes too thick, its ability to circulate becomes impaired. This can greatly increase the risk of serious thrombic event including embolism and stroke. A high hematocrit level is also an independent risk factor for heart disease.127 The normal hematocrit level in men is 40.7 to 50.30/0, and in women it is 36.1 to 44.3% (numbers may vary very slightly depending on the source). For the sake of scale, while a hematocrit of 500/0 may be normal, a hematocrit of 60% or above is considered critical (life threatening).
Anabolic/steroid administration tends to raise the hematocrit level by several percentage points, sometimes more. As a result, many steroid-using bodybuilders will have hematocrit levels that are above the normal range. For example, one study measured the average hematocrit in a group of steroid abusing competitive bodybuilders to be 55.70/0.128 This level is considered clinically high, and would increase blood viscosity enough to raise the risk of serious cardiovascular event. Although not likely to be an isolated cause, high hematocrit is believed to have been a contributing factor in the deaths of a number of steroid abusers, usually paired with high blood pressure, homocysteine, and/or atherosclerosis. The average hematocrit level in bodybuilders not taking anabolic/androgenic steroids was 45.6%, well within the normal range for healthy adult men.
Many physicians that specialize in hormone replacement therapy consider a hematocrit level of 550/0 to be an absolute cutoff point. At or above this point, and anabolic/androgenic steroid therapy cannot be continued safely. Drug intake would be ceased at this point until the hematocrit issues have been corrected. Minor elevations in hematocrit may be addressed with phlebotomy. For this, 1 pint of blood may be removed periodically during steroid intake, often every two months. Proper hydration is also important, as dehydration can temporarily cause the hematocrit level to elevate, giving a false positive for polycythemia. The daily intake of aspirin is also commonly advised if the hematocrit is above normal, as this will reduce platelet aggregation, or the tendency for platelets to stick together and form clots. Individuals remain cautioned of the potential cardiovascular danger of high hematocrit levels associated with anabolic/androgenic steroid use.
Homocysteine
Anabolic/androgenic steroids may elevate homocysteine levels. Homocysteine is an intermediary amino acid produced as a byproduct of methionine metabolism. High levels of homocysteine have been linked to elevations in the risk for cardiovascular disease.129 It is believed to play a direct role in the disease, increasing oxidative stress, including the oxidation of LDL cholesterol, and accelerating atherosclerosis.13o Elevated levels of homocysteine may also induce vascular cell damage, support platelet aggregation, and increase the likelihood of thrombic event.131 132 133 The normal range for homocysteine levels in men aged 30 to 59 years is 6.311.2umoIlL. For women of the same age the average is 4.5-7.9umol/L. Increased risk of heart attack, stroke, o~ other thrombic event are noted with even modest elevations in homocysteine. According to one study, 2 homocysteine level exceeding 15umollL in patients wit~ heart disease is associated with a 24.70/0 increasec likelihood of death within five years .
Androgens' stimulate elevations in homocysteine,135 an~ men have an approximately 250/0 higher level on averag~ than women.136 Anabolic/androgenic steroid abuse hal been associated with hyperhomocysteinaemia, 0 consistent clinically high homocysteine levels.137 Oni study found that the average homocysteinJ concentration in a group of 10 men that had been sel~
I
administering anabolic/androgenic steroids (in a cyclij pattern) for 20 years was 13.2 umol/L.138 Three of thes\ men died of a heart attack during the investigation, an~ had homocysteine levels between 15umol/L an~ 18umollL. The average homocysteine level i~li bodybuilders who had never taken steroids wa 8.7umol/L, while it was 10.4umol/L in previous steroid· users (3 months abstinence). One study did show thai administering 200 mg of testosterone enanthate (Wit~ and without an aromatase inhibitor) for three weeks faile~ to produce a significant elevation in homocysteine.139 It il unknown if the moderate dosage, drug type (esterifie~')injectable vs. c17-aa), or short duration of intake were factors in the differing outcome from other studies. Individuals remain warned of the potential for elevations in the homocysteine level with steroid abuse.
Vascular Reactivity
The endothelium is a layer of cells that line the entire circulatory system. These cells are found on the inside of all blood vessels, and help increase or decrease blood flow and pressure by relaxing or constricting the vessels (referred to as vasodilation and vasoconstriction, respectively).These cells also he'lp regulate the passage of materials in and out of blood vessels, and are involved in a number of important vascular processes including blood clotting and new blood vessel formation. Having a more flexible (reactive) endothelium is generally considered desirable for health, and, likewise, the endothelium is often compromised in individuals with cardiovascular disease. Patients with endothelial dysfunction tend to notice greater vasoconstriction, restricted blood flow, higher blood pressure, local inflammation, and reduced circulatory capacity.140 This may place them at greater risk for heart attack, stroke, or thrombosis (blood clot).
Endothelial cells are androgen responsive, which may partly account for men exhibiting less vascular reactivity than women.141 Similarly, anabolic/androgenic steroid use has been shown to impair endothelial activity and vascular reactivity. Studies at the University of Innsbruck in Austria compared the level of endothelial dilation in 20 steroid users to a group of control athletes.142 Those individuals using anabolic steroids noticed slight but measurably impaired vascular dilation and endothelial function. Additional studies at the University of Wales in Cardiff comparing vascular dilation in active, previous, and non-steroid users, also demonstrated anabolic steroids to cause a decline in endothelial-independent vasodilation.143 These effects leave the steroid user with more relative "stiffness" in the vascular system, which could increase the chance of an adverse cardiovascular event. In both studies, vascular reactivity improved after the discontinuance of anabolic/androgenic steroids.
Immune System
The human immune system is responsive to sex hormones. This results in functional differences in immunity between the sexes. Women tend to have a more active immune system compared to men, and are slightly more resistant to bacterial infection and other types of infection.144 The female immune system is also more prone to developing autoimmune diseases, which may be linked to its higher level of activity.145 The day-to-day activity of the immune system can also fluctuate throughout the menstrual cycle, further demonstrating thestrong influence ofsexsteroids.146Theslightlyweaker resistance to infection of men appears to be caused by testosterone, which is an immunosuppressive hormone.147 Androgens may modulate the immune system directly, through their conversion to estrogens,148 or by modifying glucocorticoid activity.
Anabolic/androgenic steroids have displayed both immunostimulatory and immunosuppressive actions in animal models.15o Given that these drugs can influence the immune system through a variety of pathways, and anabolic steroids are a fairly diverse class of drugs, their effects on the immune system may vary depending on the particular conditions. When used therapeutically, changes in immune system functioning are usually minor, and have not amounted to strong immunostimulation or immunosuppression. Anabolic/androgenic steroids have also been used safely in many immunocompromised patients, such as those with muscle wasting associated with HIV infection, without any significant change in immune system or viral markers.
The use of anabolic/androgenic steroids in supratherapeutic doses may slightly impair immune system functioning, reducing an individual's resistance to certain types of infection. In one study, steroid abusers were shown to have lower serum levels of IgG, IgM, and IgA immunoglobulins (antibodies) compared to bodybuilding controls, consistent with immunosuppression.153 Although this may logically increase the chance of contracting certain types of illness a significant increase in the history of illness could not be established in these same steroid abusers. Given the very random nature of illness, however, it may be difficult to establish such a link without extensive study. The effect of hormone manipulation on immunity should also be temporary, and return to a normal state once pre-treated hormonal chemistry is restored. Individuals remain warned of the potential for minor immunosuppression and increased chance of illness with steroid abuse.
Kidlleys (Renal System)
Anabolic/androgenic steroids are generally well tolerated by the renal system.These drugs are largely excreted from the body through the kidneys, although there is no inherent strong toxicity in this process. In fact, there are many instances in which these drugs may be used as supportive treatment in patients with compromised kidney function. For example, anabolic steroids have been prescribed to increase the production of red blood cells in' patients with anemia related to various forms of kidney, disease.154 155 They have even been used as general anabolic (lean body mass) support, and to treat hypogonadism, in patients undergoing dialysis.156 157 While care must be taken with such patients, therapy maYl often be conducted very safely. In otherwise healthy: individuals, clinical renal toxicity caused by the short-term administration of anabolic/androgenic steroids is unlikely.
There have been isolated reports of severe kidney damage in steroid abusers. For example, a handful ot individuals have developed Wilms' Tumo~ (nephroblastoma),158 159 which is a rare form of kidne~ cancer usually found in children. Its appearance in adul1: steroid users is suspect, but not conclusive evidence tha1 drugs were the actual cause.There have also been isolate~ reports of renal cell carcinoma in steroid abusers.160 16] Since this is the most common form of kidney cance~ however, conclusive links are again difficult to draw.Ther~: have additionally been case reports of combined liver an~ renal failure with steroid abuse.162 163 In these casel kidney failure may have been subsequent to steroid induced liver toxicity, as cholestasis (bile duct obstructio~ is known to cause acute tubular necrosis and renal failure.
Kidney health should be a concern for long-term steroid! using bodybuilders and power athletes. To begin with excessive resistance training can produce some strain o~ the renal system. A condition called rhabdomyolysis i~ caused by the extreme damage of muscle tissue, whic~ releases myoglobin and a number of nephrotoxi1 compounds into the blood. In high levels this ca~ damage kidney tissue and even cause renal failure. Ther~' have been rare case reports of severe clinicarhabdomyolysis in bodybuilders, both with and without mentionofsteroid abuse. Steroid use mayalso cause hypertension, which can lead to kidney damage.17o While anabolic/androgenic steroids are generally not regarded as direct kidney toxic drugs, they may be used to support a lifestyle and long-term metabolic state characterized by extreme training, heightened daily muscle protein turnover, and elevated blood pressure. Over time this may compromise kidney health. Regular monitoring of kidney function is recommended.
Liver (Hepatic System)
Many oral anabolic/androgenic steroids (or injectable forms of oral steroids) are toxic to the liver (hepatotoxic). These compounds can cause serious and sometimes lifethreatening damage when abused, and occasionally even under therapeutic conditions. Those agents commonly associated with clinical hepatotoxicity include (but are not limited to) fluoxymesterone, methandrostenolone, methylandrostenediol, methyltestosterone, norethandrolone, oxymetholone, and stanozolol.171 172 173 174 175 These steroids all have either an ethyl or methyl group at carbon-17 (c-17alpha alkylation). All c-17alpha alkylated anabolic/androgenic steroids possess some level of hepatotoxicity. Liver strain, as assessed by elevated liver enzymes, has also been reported with non-alkylated esterified injectable steroids including nandrolone decanoate and testosterone enanthate in extremely rare instances.176 177 These steroids have never been associated with serious hepatic damage, however, and are not regarded as liver toxic.
Alkylation of c-17alpha specifically protects the steroid molecule from metabolism by the enzyme 17betahydroxysteroid dehydrogenase (17beta-HSD). This enzyme normally oxidizes a steroid's 17beta-hydroxyl (17beta-ol) group, which must remain intact for the drug to impart any anabolic or androgenic effect. Oxidation of 17-beta-ol is one of the primary pathways of hepatic steroid deactivation. Without protection from this enzyme, very little active drug will survive the first pass through the liver and reach circulation after oral dosing. Alkylation of c-17alpha effectively protects the steroid from 17beta-HSD by occupying a hydrogen bond necessary for the breakdown of 17beta-ol to 17-keto. The compound must be metabolized through other pathways as a result, and immediate hepatic deactivation is prevented. The process allows a very high percentage of the steroid dose to pass into the bloodstream intact, but it also places some strain on the Iiv~r in the process.
The exact mechanism of hepatotoxicity induced by alkylated anabolic/androgenic steroids remains unknown, but it is speculated to be due in large part to the natural activity of androgens in the liver. This liver possesses a high concentration of androgen receptors, and is responsive to these hormones.178 With physiological androgens such as testosterone and dihydrotestosterone, however, only a moderate level of activity is permitted in this organ. This is because the liver is normally very efficient at metabolizing steroids, which mutes their local activity. But with the liver unable to easily deactivate alkylated steroids, however, a far greater level of hepatic androgenic activity is allowed. The concentration of steroid in the liver is also very high after oral administration, as the digestive tract delivers the drug directly to this organ before it can reach circulation. The fact that the most potent steroid ever given to humans on a mg-for-mg basis is also the most liver toxic, also supports a close association between androgenic potency and hepatotoxicity.
Early liver toxicity is usually visible in blood test results for hepatic function before physical symptoms or dysfunction develop. This is most likely to include elevations in aminotransferase enzymes AST and ALT, also called serum glutamic-oxalocetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT), respectively. The cholestatic enzymes alkaline phosphatase (ALP) and gamma-glutamyltranspeptidase (GGT) may also be elevated, along with other markers (see: Understanding Blood Tests). Screening for abnormalities in hepatic markers is regarded as the most effective way of preventing liver damage from steroid administration. Should asymptomatic toxicity go unnoticed and without a change in drug intake, it is likely to progress to more severe hepatic strain, injury, or hepatic dysfunction. Immediate cessation of anabolic/androgenic steroid use and a full assessment of liver and full-body health is advised should any signs of unacceptable liver toxicity become apparent.
The most common form of actual liver dysfunction caused by the administration of oral anabolic/androgenic steroids is cholestasis.181 This describes a condition where the flow of bile becomes decreased, usually because of obstruction of the small bile ducts in the liver (intrahepatic). This causes bile salts and bilirubin to accumulate in the liver and blood instead of being properly excreted thorough the d'igestive tract. Inflammation (hepatitis) may also be present.182 Symptoms of cholestasis may include anorexia, malaise, nausea, vomiting, upper abdominal pain, or pruritus (itching). The stool may also change to a clay color (alcholic stool) due to the reduced excretion of bile, and the urine may become amber. Cholestatic jaundice may develop, which is characterized by a yellowing of the skin, eyes, and mucous membranes due to high levels of bilirubin in the blood (hyperbilirubinemia). Intrahepatic cholestasis may also coincide with hepatocellular necrotic lesions (death of liver tissue).
Intrahepatic cholestasis will usually resolve itself without serious injury or medical intervention within several weeks of discontinuing all hepatotoxic steroids. More serious cases may take several months before normal hepatic enzyme levels and functioning are restored. Hepatic lesions are likely to heal over time as well, at least partially. In some cases physicians have initiated supportive treatment with ursodeoxycholic acid (ursodiol), which is a secondary bile salt known to possess hepatoprotective and anti-cholestatic effects, in an effort to hasten recovery.183 The exact value of using this medication to treat steroid-induced cholestatic jaundice remains unknown, however. The liver is highly resilient, and intrahepatic cholestasis is unlikely to continue degrading after drug discontinuance unless additional pathologies are present.
More serious hepatic complications are rare, but have included peliosis hepatis184 (blood-filled cysts on the liver), portal hypertension with variceal bleeding 185 (bleeding caused by increased blood pressure in portal vein related to obstructed blood flow), hepatocellular adenoma186 (non-malignant liver tumor), hepatocellular carcinoma 187 (malignant liver tumor), and hepatic angiosarcoma 188 (aggressive malignant cancer of the lining of blood vessels inside the liver). Some of these pathologies can be very insidious at times, developing quickly and without clear early symptoms. Although many of these potentially life-threatening side effects have often been attributed to very ill patients receiving steroid medications, a growing number of case reports are now involving otherwise healthy young bodybuilders abusing these drugs.
Physical
Acne : Androgens stimulate the sebaceous glands in the skin to secrete an oily substance called sebum, which is made of fats and the remnants of dead fat-producing cells. Excess stimulation, as with steroid abuse, may also cause a significant increase in the size ofthe sebaceous glands.191 Sebaceous glands are found at the base of the hair follicles in all hair-containing areas of the skin. If the androgen level becomes too high and the sebaceous glands become overactive, the hair follicles may begin to clog with sebum and dead skin cells, resulting in acne Acne vulgaris (common acne) is frequent in steroid users, especially when the drugs are taken in supratherapeutic levels. This often includes acne lesions on the face, back, shoulders, and/or chest.
A mild incidence of acne vulgaris is usually addressed with topical over-the-counter acne medications and a rigorous skin cleaning routine that removes excess oil and dirt. More serious acne may develop in sensitive individuals, including acne conglobata (severe acne with connected nodules under the skin) or acne fulminans (highly destructive inflammatory acne). Such incidences may require medical intervention, which usually involves treatment with isotretinoin. Topical anti-androgen drugs are also under investigation for the treatment of severe acne, and have shown a great deal of promise in earl} trials.192 Acne is typically resolved with the cessation 01 steroid use, although the overproduction of sebum ma} persist until the sebaceous glands have had time tc atrophy back to original size. Serious forms of acne ma} produce lasting scars.
Hair Loss (Androgenetic Alopecia)
Anabolic/androgenic steroids may contribute to a form d, hair loss on the scalp known as androgenetic alopeci'\ (AGA). This disorder is characterized by a progressivl! miniaturization of hair follicles, and a shortening of th anagen phase of hair growth, under androgen influence The hair produced by affected follicles will progressivelj thin, covering the scalp less and less effectively. In meH the baldness produced is usually identified most simply aJ male pattern. This will initially include a receding hairlinJ . (fronto-temporal thinning) and thinning on the crowrl areas where androgen receptor concentrations are higH In women, the balding usually takes on a more diffuse pattern, with thinning throughout the top of the head. Most women with androgenetic alopecia do not have a receding hairline.
Androgenetic alopecia is the most common form of hair loss in men and women alike. It is especially common in males, and more than S09tb of the population will notice it by the age of 50.193 As its name signifies, androgenetic alopecia involves the interplay of both androgenic hormones and genetic factors. Individuals with this condition appear to be more locally sensitive to androgens, and have higher levels of androgen receptor protein and dihydrotestosterone in the scalp, in comparison to those unaffected.194 Although dihydrotestosterone is identified as the primary hormone involved in the progress of androgenetic alopecia, it does not possess a unique ability to influence this condition. All anabolic/androgenic steroids stimulate the same cellular receptor, and as a result are capable of providing the necessary androgenic stimulation. Baldness can result from steroid use, even in the absence of steroids that convert to, or are derived from, dihydrotestosterone.
The genetics of androgenetic alopecia are not fully understood. At one time it was believed this condition could be inherited solely from the maternal grandfather. More recent evidence contradicts this notion, however, showing strong support for father-to-son transmission in many cases.195 A number of genes have been identified as having a potential link to the disorder, including certain variants (polymorphisms) of the androgen receptor gene.l96 197 No single genetic variant alone has yet been able to explain all cases of androgenetic alopecia, however. AGA is now believed to involve several genes (polygenic).198 The way these genes combine, and the level of androgens in the scalp, may ultimately work together to control the onset and severity of androgenetic alopecia. Estrogen is also known to lengthen the anagen phase,199 and the pathogenesis of this condition may ultimately involve genes that alter both androgen and estrogen activity.
Treatment for androgenetic alopecia in men usually involves topical minoxidil and oral finasteride, as-alpha reductase enzyme inhibitor. Women are typically prescribed anti-androgens and estrogen/progestin drugs. The focus in both cases is on reducing relative androgenic action in the scalp, which may (at least temporarily) stall the condition. With this in mind, many steroid users concerned with hair loss will tailor their drug intake to minimize unnecessary androgenic activity. This usually involves moderate dosing and the careful selection of drugs with high anabolic-to-androgenic ratios, such as oxandrolone, methenolone, or nandrolone. Alternatively, some may choose to use injectable testosterone esters combined with finasteride to reduce scalp DHT conversion. These strategies are met with varying degrees of success.
There has been no study on the role of genetics in baldness linked to steroid abuse. Anecdotally, individuals with existing visible androgenetic alopecia appear to be those most susceptible to the effects of anabolic/androgenic steroids on the scalp. For many of these people, the loss of hair appears significantly accelerated when taking these drugs. On the other hand, this side effect is generally a much less significant issue with individuals that have not noticed thinning beforehand. Many go on to abuse steroids for years without any visible effect at all, making it clear that there is more to this disorder than local androgen levels. It is well understood that androgens play a role in the progression of androgenetic alopecia for those genetically prone. Steroid use can, therefore, coincide with the first noticeable onset of this condition. It is unknown, however, if anabolic/androgenic steroid abuse can cause baldness in an individual that does not carry any genetic susceptibility.
Water and Salt Retention
Anabolic/androgenic steroids may increase the amount of water and sodium stored in the body. This may include increases in both the intracellular and extracellular water compartments. Intracellular fluid refers to water that has been drawn inside the cells. While this does not increase the protein content of the muscles, it does expand the muscle cell, and is often calculated and viewed as a part of total fat free body mass. Extracellular water is stored in the circulatory system, as well as in various body tissues, in the spaces between cells (interstitial). Increases in interstitial fluid can be noticeable and troubling cosmetically. In strong cases this can bring about a very puffy appearance to the body (peripheral or localized edema), with bloating of the hands, arms, body, and face. This may reduce the visibility of muscle features throughout the physique. Excess fluid retention can also be associated with elevated blood pressure/os which can increase cardiovascular and: renal strain.
Estrogen is a regulator of fluid retention in both men and women.206 This effect appears to be mediated in part by: changes in hypothalamic arginine vasopressin (AVP), the: primary hormone involved in controlling water reabsorption in the kidneys.207 Increased levels 01 estrogen tend to increase AVP levels, which can promote the increased storage of water. Estrogen also appears td act on the renal tubes in the kidneys in an aldosterone~ independent manner to increase the reabsorption 01 sodium.20B Sodium is the major electrolyte in th~ extracellular environment, and helps to regulate thJ osmotic balance of cells. Higher levels can significantl)1 increase water in the extracellular compartmen~ Anabolic/androgenic steroids that either convert t~ estrogen, or possess inherent estrogenic activity, arei likewise, those steroids that are associated with increaseq extracellular water retention.20g
Estrogenic anabolic/androgenic steroids are generall) favored for mass gaining (bulking) purposes. A steroid\ user may ignore water retention during this phase o~ training, occasionally even finding the sheer increases in size to be a welcome benefit. Estrogenic steroids such aSl testosterone and oxymetholone are also regarded as theIl strongest mass-and strength-building agents, which may] be caused in part by anabolic benefits of elevated estrogenic activity.The excess water stored in the muscles'lll joints, and connective tissues is also commonly believed
to increase an individual's resistance to injury.With the use of many strongly estrogenic anabolic/androgenic steroids, water retention can account for a large portion (350/0 or more) of the initial body weight gain during steroid treatment. This weight is quickly lost once the steroids are discontinued or estrogenic activity is reduced.
Non-aromatizing steroids such as oxandrolone and stanozolol have also been shown to promote increased water retention, so this effect is not entirely exclusive to aromatizable or estrogenic substances.210 211 Anabolic steroids with low or no estrogenic action tend to produce modest increases in whole body water and intracellular fluid retention, however, and not in the visible extracellular compartment.212 213 These steroids are considered to be more cosmetically appealing, and are generally favored by bodybuilders and athletes when looking to improve lean mass and muscle definition. Popular anabolic/androgenic steroids that are associated with low visible water retention include fluoxymesterone, methenolone, nandrolone, oxandrolone, stanozolol, and trenbolone.
Excess water retention may be addressed with the use of ancillary medications such as the anti-estrogen tamoxifen citrate, or an aromatase inhibitor such as anastrozole. By minimizing the activity of estrogens, these drugs can effectively reduce the level of stored water. In most cases where an aromatizable steroid is used, aromatase inhibitors prove to be significantly more effective at achieving this goal. A common practice among bodybuilders during competition is to also use a diuretic, which can shed excess water by directly increasing renal water excretion. This is regarded as the most effective method for rapidly improving muscle definition, but it can also be one of the most acutely risky practices as well. Water retention is not a persistent side effect of steroid use. Excess water is quickly eliminated, and normal water balance returned, once anabolic/androgenic steroid administration is halted.
Dysphonia (Vocal Changes)
Although far less common than dysphonia in women, anabolic/androgenic steroids may alter vocal physiology in men. This may include a deepening of the voice. Dysphonia is most common when anabolic/androgenic steroids are administered during adolescence, as the deeper adult voice has not yet been established under the influence of androgens. The administration of anabolic/androgenic steroids before maturity can, likewise, cause a progressive lowering of the vocal pitch, and may trigger pubescent vocal changes in younger patients. Androgens have much less (often minimal) effect on vocal physiology in adulthood. Although a slight lowering of the voice may be noticed with androgen use in some cases, reports of clinically significant changes in the vocal quality of adult men are, likewise, very rare.There has also been an isolated report of stridor (vibrating noise when breathing) and vocal hoarseness in relation to anabolic/androgenic steroid abuse.214 This instance also involved smoking, however, making the direct influence of steroids more difficult to discern. In general, vocal physiology is well established by adulthood. Aside from very minor reductions in pitch, anabolic/androgenic steroids are not expected to have strong audible effects on the voice.
Gynecomastia
Anabolic/androgenic steroids with significant estrogenic or progestational activity may cause gynecomastia (female breast development in males). This disorder is specifically characterized by the growth of excess glandular tissue in men, due to an imbalance of male and female sex hormones in the breast. Estrogen is the primary supporter of mammary gland growth, and acts upon receptors in the breast to promote ductal epithelial hyperplasia, ductal elongation/branching, and fibroblast proliferation.215 Androgens, on the other hand, inhibit glandular tissue growth.216 High serum androgen levels and low estrogen usually prevent this tissue development in men, but it is possible in both sexes given the right hormonal environment. Gynecomastia is regarded as an unsightly side effect of anabolic/androgenic abuse by most users. In extreme cases the breast may take on a very female looking appearance, which is difficult to hide even Physical (Female) with loose clothing Gynecomastia tends to develop in a series of progressive Birth Delects
stages. The severity of this process will vary depending on the type and dose of drug(s) used, and individual sensitivity to hormones. The first sign is typically pain in the nipple area (gynecodynea). This may quickly coincide with minor swelling around the nipple area (Iipomastia). This is sometimes referred to as pseudo-gynecomastia, as it primarily involves fat and not glandular tissue. At this stage, it may be possible to address mild nipple swelling by reducing or eliminating the offending steroidal compounds, and administering an appropriate antiestrogenic medication for several weeks. If left untreated, however, this may quickly progress to clear gynecomastia, which involves significant fat, fibrous, and glandular tissue growth. The hard tissue growth may be easily felt in the early stages when pinching deeply around the nipple. Noticeable gynecomastia is likely to require corrective cosmetic surgery (male breast reduction).
Although gynecomastia is a very common side effect of steroid abuse, given its clear association with certain drugs or practices, it is also an easily avoidable one. Careful steroid selection and reasonable dosing are usually regarded as the most basic and reliable methods for preventing its onset. Many steroid users also frequently take some form of estrogen maintenance medication, which may effectively counter the effects of elevated estrogenicity. Common options include the anti-estrogen tamoxif~n citrate, or an aromatase inhibitor such as anastrozole. The use of a post-cycle hormone recovery program at the conclusion of steroid administration (which usually includes several weeks of anti-estrogen use) is also commonly advised, as gynecomastia is sometimes reported in the post-cycle hormone imbalance phase when steroids are not actually being taken.
It is important to note that progesterone can also augment the stimulatory effect of estrogen on mammary tissue growth.218 As such, progestational drugs may be able to trigger the onset of gynecomastia in sensitive individuals, even without elevating levels of estrogen. Many anabolic steroids, particularly those derived from nandrolone, are known to exhibit strong progestational activity. While gynecomastia is not a common compliant with these drugs, they are occasionally linked to this side effect in anecdotal reports. The anti-estrogen tamoxifen citrate is usually taken in such instances, as it can offset the effects of estrogen at the receptor, which are still necessary for progestins to impart their growthpromoting effects on the breast.


